Safety: Microbial Testing
Safety Profile
The primary safety benefit of the Theraworx range of products is to the patient. The reference documents include results of cytotoxicity, irritant and biocompatibility testing. Avadim has tested the safety profile of Theraworx versus CHG in mucosal tissue, and those results are also included in the reference documents.The advantage of having a skin-friendly product which helps protect the natural microbiome through its low pH characteristics, and is also safe for use in all body areas, while still having significant effectiveness, is a key part of why we feel that the product represents a new and innovative technology. Having effective potency, and non-toxic biocompatibility, at the same time represents unique skin hygiene technology. Compared to CHG, Theraworx presents an alternative that mitigates potential concerns of nurses regarding irritation or allergic reactions.
ISSUED 6/12/08 REV 7-8-14
1.0 Objective
• 1.1 The objective of this summary is to briefly delineate the scope of testing that has been conducted to assure the safety and effectiveness of the Theraworx platform.
2.0 Scope
• 2.1 The scope of this report shall cover the original testing completed as part of the patented process (2002), as well as all subsequent antimicrobial and biocompatibility testing recently conducted.
3.0 References
• 3.1 AATCC Method 100: Antibacterial Finishes on Textile Materials: Assessment of
• 3.2 ISO/DIS 10993: Biological evaluation of Medical Devices – Part 1: Evaluation and Testing
4.0 Attachments
• 4.1 Test reports available upon request.
5.0 Definitions
• 5.1 Theraworx – A Patented, pH and Hygiene Management System.
6.0 Testing Data
• 6.1Original Testing
• 6.1.1 Antimicrobial Activity Testing – Testing was conducted by Microbiological Consultants, Inc. on behalf of Harod Enterprises, Inc. on January 15, 1998.
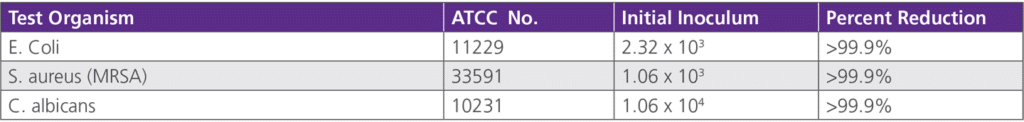
• 6.1.2 Antimicrobial Efficacy TestingTesting was conducted by Microbiological Consultants, Inc. on behalf of Harod Enterprises, Inc. on June 12, 2000

• 6.1.3 Antiviral Efficacy TestingTesting was conducted by Microbiological Consultants, Inc. on behalf of Harod Enterprises, Inc. on July 3, 2000.

• 6.2 Antimicrobial Testingtion
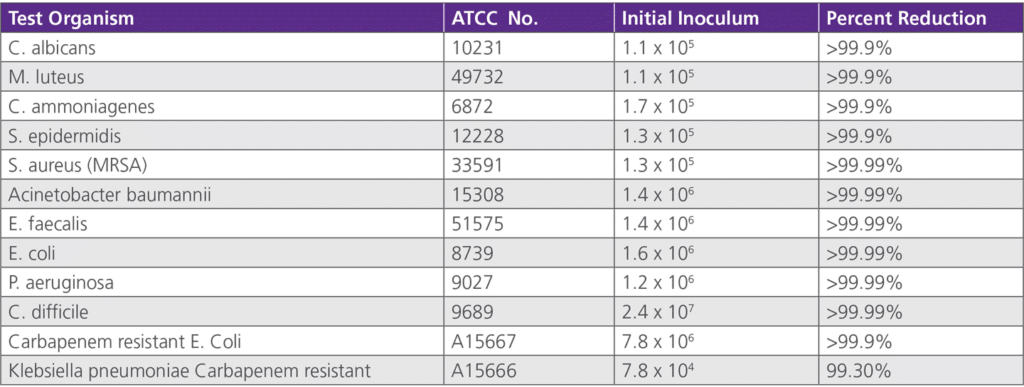
• 6.2.1 AATCC Method 100 Antimicrobial Testing – Testing was conducted by Apptec Laboratories on behalf of Avadim, LLC between September 5, 2007 throughJune 12, 2008
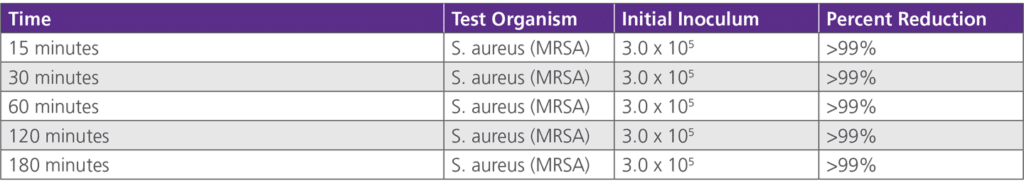
• 6.2.2 Antimicrobial Efficacy Duration Study – Testing was conducted by St. John’s Research Institute on behalf of Avadim, LLC on November 14, 2007. This study was based upon a one-time application of collegen and re-inoculated at various time periods.on
• 6.3 Biocompatability Testing
• 6.3.1 Outline Theraworx has been subject to in vitro and in vivo biocompatibility testing (ISO Intracutaneous Reactivity Test,ISO Acute Systemic Injection Test, ISO Guinea Pig Maximization Sensitization Test, and MEM-Elution using L-929 Mouse Fibroblast Cells(ISO) (Cytotoxicity)). These tests support the safe use of Theraworx in contact with breached or compromised skin.
• 6.3.2 MEM Elution Using L-929 Mouse Fibroblast Cells (ISO) (Cytotoxicity)
• 6.3.1.1 Reference: ISO 10993-1
• 6.3.1.2 Report No. 66958
• 6.3.1.3 Date Tested: November 9,2007
• 6.3.1.4 Conducted By: Apptec Laboratories
• 6.3.1.5 Results: Test was considered valid as the control results were within acceptable parameter. The test article PASSED and is considered NON-TOXIC under the test conditions employed.
• 6.3.3 ISO Intracutaneous Reactivity Test
• 6.3.2.1 Reference: ISO 10993-1
• 6.3.2.2 Report No. 66959
• 6.3.2.3 Date Tested: December 10,2007
• 6.3.2.4 Conducted By: Apptec Laboratories
• 6.3.2.5 Results: The test article is considered a NON-IRRITANT.
• 6.3.4 ISO Acute Systemic Injection Test
• 6.3.3.1 Reference: ISO 10993-1
• 6.3.3.2 Report No. 101611
• 6.3.3.3 Date Tested: February 11,2008
• 6.3.3.4 Conducted By: Apptec Laboratories
• 6.3.3.5 Results: No potential toxic effects as a result of a single-dose systemic injection were observed;test article PASSED the test.
• 6.3.5 ISO Guinea Pig Maximization Sensitization Test
• 6.3.4.1 Reference: ISO 10993-1
• 6.3.4.2 Report No. 101612
• 6.3.4.3 Date Tested: March 13, 2008
• 6.3.4.4 Conducted By: Apptec Laboratories
• 6.3.4.5 Results: None of the test animals challenged with the test article extracts were observed with a sensitization response greater than “0”. Test article did NOT elicit a sensitization response.
